#computational genomics
Explore tagged Tumblr posts
Text
https://justpaste.it/f7dbu
#bioinformatics projects#ai bioinformatics#genomics in bioinformatics#microarray in bioinformatics#computational genomics#computational genetics#bioinformatics services#bioinformatics analysis of ngs data
0 notes
Text

Baby Blue a prototype automated thermal cycler for PCR (1986)
339 notes
·
View notes
Text
As much as I dislike how science works, I love all the public data available in databases! It's pretty cool being able to download and see on your own computer a 10000 yr old guy's genome sequence
Cheddar man my beloved <3
This is a piece of FOXP2, a gene involved in speech and language development, very important for our ability to speak.
Article that sequenced his genome: https://www.nature.com/articles/s41559-019-0871-9 (unfortunately not public 😔)
ENA project ID: PRJEB31249 (https://www.ebi.ac.uk/ena/browser/view/PRJEB31249?show=reads) if you want to download it it's the SB524A to SB524A8 BAM & BAI files, it's a bit tricky to find on the paper.
#you don't even need a powerful computer to do it mine certainly isn't#just a few gb of space#genomes are heavy#genomics#science#biology
9 notes
·
View notes
Text
Guys who work in bioinformatics and come from biology studies: how do you handle lack of inspiration problems? I like to code i find it very creative and genomics is an area that can help today's scientists answer many questions but i miss drawing anatomic parts and learning about fun molecules and pathways that was a big part of my joy during my undergraduate studies. Do you have any suggestions on this? Im currently reading Austin's Kleon book steal like an artist and he proposes having two desks one analog and one digital and work mostly on analog and edit final work on digital. But i struggle find a way to apply this in everyday tasks like NCBI data upload or run jobs in a cluster. Any thoughts?

#grad school#gradblr#phdblr#studyblr#study motivation#studyspo#academia#genomics#study notes#bioinformatics#computer science#creativity#ideas#inspiration
11 notes
·
View notes
Note
Hii how are you doing!!!
You're taking Bioinformatics too right? What resources are you using to understand it HELP—
Hii I'm good, thanks! Hbu? That's so cool you're self-studying bioinformatics too! You're coming from the bio side as well, right?
I haven't really gotten into actual bioinformatics stuff yet bc last I tried, I was overwhelmed by all the info I was expected to know...biology, stats, and coding/computer science stuff. I feel I still need to know more on all of those fronts, but coming from a life science background, the part that's most foreign to me is the coding aspect, so I'm trying to work through an intro to computer science mooc by Harvard called cs50x and figure out where to go from there... Also, the codeblr community has been nothing but extremely encouraging and helpful. Many have compiled resources for learning to code like in this post.
Sorry if this wasn't very helpful, I'm at the very very very beginning in this 😅 Maybe once I'm further along, I'll know enough to make a masterpost of resources I used and found helpful!
If anyone has any other suggestions, please feel free to add in the reblogs!
#stemblr#studyblr#chemblr#stem academia#stem student#codeblr#progblr#bioinformatics#computational biology#asks#mittonstudies#heyfrithams#heyharri#heydilli#heyzainab#benniscup#myhoneststudyblr#genomics#bioblr
21 notes
·
View notes
Text
Scientists unveil genome-driven imaging for medical diagnosis

- By InnoNurse Staff -
Methods of imaging like computed tomography (CT) and positron emission tomography (PET) are crucial in diagnosing and pinpointing various illnesses. A recently devised approach allows PET to specifically leverage alterations in the human genome for diagnosis.
Read more at Universität Luzern/Medical Xpress
#imaging#genomics#dna#medtech#health tech#computed tomography#positron emission tomography#medical imaging#diagnostics
2 notes
·
View notes
Text
Killing the messenger
New Post has been published on https://thedigitalinsider.com/killing-the-messenger/
Killing the messenger


Like humans and other complex multicellular organisms, single-celled bacteria can fall ill and fight off viral infections. A bacterial virus is caused by a bacteriophage, or, more simply, phage, which is one of the most ubiquitous life forms on earth. Phages and bacteria are engaged in a constant battle, the virus attempting to circumvent the bacteria’s defenses, and the bacteria racing to find new ways to protect itself.
These anti-phage defense systems are carefully controlled, and prudently managed — dormant, but always poised to strike.
New open-access research recently published in Nature from the Laub Lab in the Department of Biology at MIT has characterized an anti-phage defense system in bacteria, CmdTAC. CmdTAC prevents viral infection by altering the single-stranded genetic code used to produce proteins, messenger RNA.
This defense system detects phage infection at a stage when the viral phage has already commandeered the host’s machinery for its own purposes. In the face of annihilation, the ill-fated bacterium activates a defense system that will halt translation, preventing the creation of new proteins and aborting the infection — but dooming itself in the process.
“When bacteria are in a group, they’re kind of like a multicellular organism that is not connected to one another. It’s an evolutionarily beneficial strategy for one cell to kill itself to save another identical cell,” says Christopher Vassallo, a postdoc and co-author of the study. “You could say it’s like self-sacrifice: One cell dies to protect the other cells.”
The enzyme responsible for altering the mRNA is called an ADP-ribosyltransferase. Researchers have characterized hundreds of these enzymes — although a few are known to target DNA or RNA, all but a handful target proteins. This is the first time these enzymes have been characterized targeting mRNA within cells.
Expanding understanding of anti-phage defense
Co-first author and graduate student Christopher Doering notes that it is only within the last decade or so that researchers have begun to appreciate the breadth of diversity and complexity of anti-phage defense systems. For example, CRISPR gene editing, a technique used in everything from medicine to agriculture, is rooted in research on the bacterial CRISPR-Cas9 anti-phage defense system.
CmdTAC is a subset of a widespread anti-phage defense mechanism called a toxin-antitoxin system. A TA system is just that: a toxin capable of killing or altering the cell’s processes rendered inert by an associated antitoxin.
Although these TA systems can be identified — if the toxin is expressed by itself, it kills or inhibits the growth of the cell; if the toxin and antitoxin are expressed together, the toxin is neutralized — characterizing the cascade of circumstances that activates these systems requires extensive effort. In recent years, however, many TA systems have been shown to serve as anti-phage defense.
Two general questions need to be answered to understand a viral defense system: How do bacteria detect an infection, and how do they respond?
Detecting infection
CmdTAC is a TA system with an additional element, and the three components generally exist in a stable complex: the toxic CmdT, the antitoxin CmdA, and an additional component called a chaperone, CmdC.
If the phage’s protective capsid protein is present, CmdC disassociates from CmdT and CmdA and interacts with the phage capsid protein instead. In the model outlined in the paper, the chaperone CmdC is, therefore, the sensor of the system, responsible for recognizing when an infection is occurring. Structural proteins, such as the capsid that protects the phage genome, are a common trigger because they’re abundant and essential to the phage.
The uncoupling of CmdC exposes the neutralizing antitoxin CmdA to be degraded, which releases the toxin CmdT to do its lethal work.
Toxicity on the loose
The researchers were guided by computational tools, so they knew that CmdT was likely an ADP-ribosyltransferase due to its similarities to other such enzymes. As the name suggests, the enzyme transfers an ADP ribose onto its target.
To determine if CmdT interacted with any sequences or positions in particular, they tested a mix of short sequences of single-stranded RNA. RNA has four bases: A, U, G, and C, and the evidence points to the enzyme recognizing GA sequences.
The CmdT modification of GA sequences in mRNA blocks their translation. The cessation of creating new proteins aborts the infection, preventing the phage from spreading beyond the host to infect other bacteria.
“Not only is it a new type of bacterial immune system, but the enzyme involved does something that’s never been seen before: the ADP-ribsolyation of mRNA,” Vassallo says.
Although the paper outlines the broad strokes of the anti-phage defense system, it’s unclear how CmdC interacts with the capsid protein, and how the chemical modification of GA sequences prevents translation.
Beyond bacteria
More broadly, exploring anti-phage defense aligns with the Laub Lab’s overall goal of understanding how bacteria function and evolve, but these results may have broader implications beyond bacteria.
Senior author Michael Laub, Salvador E. Luria Professor and Howard Hughes Medical Institute Investigator, says the ADP-ribosyltransferase has homologs in eukaryotes, including human cells. They are not well studied, and not among the Laub Lab’s research topics, but they are known to be up-regulated in response to viral infection.
“There are so many different — and cool — mechanisms by which organisms defend themselves against viral infection,” Laub says. “The notion that there may be some commonality between how bacteria defend themselves and how humans defend themselves is a tantalizing possibility.”
#ADP#agriculture#author#Bacteria#bases#Biology#cascade#cell#Cells#chemical#code#complexity#Computer modeling#CRISPR#CRISPR-Cas9#defense#defenses#diversity#DNA#earth#Editing#enzyme#enzymes#Fight#Forms#gene editing#genetic#genome#growth#how
0 notes
Text
The First Experience of Tomographic Representation of an ECG-Signal
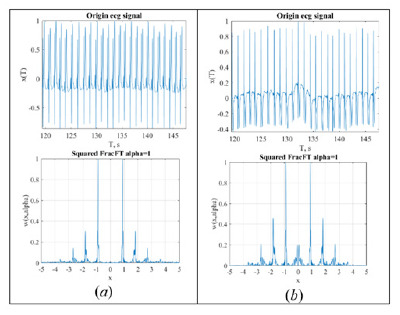
The experience for tomographic representation of ECG-signal is considered. Tomogram representation of the signal is identical to the Radon transform for processing various signals. It is shown that the tomographic representation for small parameters of transformation is identical for different leads, may reveal instability of isoelectric line and distinguish some forms of arrhythmias. This makes it possible to use only one lead for express analysis.
Read More About This Article: https://crimsonpublishers.com/oabb/fulltext/OABB.000568.php
Read More About Crimson Publishers: https://crimsonpublishers.com/oabb/index.php
#open access journals#biostatistics & bioinformatics#statistical theory#Statistical Computations#Structural genomics
0 notes
Text
How Big Data Analytics is Changing Scientific Discoveries
Introduction
In the contemporary world of the prevailing sciences and technologies, big data analytics becomes a powerful agent in such a way that scientific discoveries are being orchestrated. At Techtovio, we explore this renewed approach to reshaping research methodologies for better data interpretation and new insights into its hastening process. Read to continue
#CategoriesScience Explained#Tagsastronomy data analytics#big data analytics#big data automation#big data challenges#big data in healthcare#big data in science#big data privacy#climate data analysis#computational data processing#data analysis in research#data-driven science#environmental research#genomics big data#personalized medicine#predictive modeling in research#real-time scientific insights#scientific data integration#scientific discoveries#Technology#Science#business tech#Adobe cloud#Trends#Nvidia Drive#Analysis#Tech news#Science updates#Digital advancements#Tech trends
1 note
·
View note
Text
This Tiny Fern Has The Largest Genome Of Any Organism On Earth
— By Royal Botanic Gardens, Kew | May 31, 2024

The fern species Tmesipteris oblanceolata from New Caledonia was found to have more than 50 times more DNA in each cell than humans. According to new research, its genome size is 160.45 gigabase pairs. Credit: Pol Fernandez
In a new study published in the journal iScience, researchers from the Royal Botanic Gardens, Kew and the Institut Botànic de Barcelona (IBB-CSIC) in Spain present a new record-holder for the largest amount of DNA stored in the nucleus of any living organism on the planet.
Coming in at more than 100 meters of unraveled DNA, the New Caledonian fork fern species Tmesipteris oblanceolata was found to contain more than 50 times more DNA than humans and has dethroned the Japanese flowering plant species Paris japonica, which has held this record since 2010.
In addition, the plant has achieved three Guinness World Records titles for Largest plant genome, Largest Genome, and Largest fern genome for the amount of DNA in the nucleus.
T. oblanceolata is a rare species of fern found on the island nation of New Caledonia, an overseas French territory situated in the Southwest Pacific, about 750 miles east of Australia, and some of the neighboring islands such as Vanuatu. The genus Tmesipteris is an understudied group of plants consisting of about 15 species, most of which occur across a range of Pacific Islands and Oceania.
Until now, scientists have only estimated the size of the genomes for two species of Tmesipteris—T. tannensis and T. obliqua—both of which were found to contain gigantic genomes, at 73.19 and 147.29 gigabase pairs (Gbp) respectively.
In 2023, lead authors Dr. Jaume Pellicer and Dr. Oriane Hidalgo, from the IBB and formerly of RBG Kew, traveled to New Caledonia to collect samples of Tmesipteris, which were then analyzed to estimate the size of their genomes. This involved isolating the nuclei of thousands of cells, staining them with a dye and then measuring how much dye had bound to the DNA within each nucleus—the more dye, the bigger the genome.
youtube
The previous record holder for the world's largest genome was the flowering plant, Paris Japonica at 148.89 gigabase pairs. Credit: RBG Kew
The analysis revealed the species T. oblanceolata to have a record-breaking genome size of 160.45 Gbp, which is about 7% larger than that of P. japonica (148.89 Gbp).
When unraveled, the DNA from each cell of this fern would stand taller than the Elizabeth Tower in Westminster, London, which is 96m tall and home to the world-famous Big Ben bell. For comparison, the human genome contains about 3.1 Gbp distributed across 23 chromosomes and when stretched out like a ball of yarn, the length of DNA in each cell only measures about 2m.
Dr. Pellicer, a researcher in evolutionary biology, says, "Tmesipteris is a unique and fascinating small genus of ferns, whose ancestors evolved about 350 million years ago—well before dinosaurs set foot on Earth��and it is distinguished by its mainly epiphytic habit [it grows mainly on the trunks and branches of trees] and restricted distribution in Oceania and several Pacific Islands.
"For a long time, we thought that breaking the previous size record of Paris japonica was going to be an impossible mission, but once again, the limits of biology have surpassed our most optimistic predictions.
"Based on our previous research, we anticipated the existence of giant genomes in Tmesipteris. That said, discovering the largest genome of them all is not just a feat of scientific exploration, but the result of an almost fourteen-year journey into the boundless complexity and diversity of plant genomes."
To date, scientists across the globe have estimated the genome sizes of more than 20,000 eukaryotic organisms, revealing in the process a wide range of genome sizes across the tree of life. These, in turn, have been found to have a profound impact not only on their anatomy, as bigger genomes need bigger cells to house them and take longer to replicate, but also how they function, evolve, and where and how they live.

The DNA of T. oblanceolata measures over 106m in length, making it taller than Elizabeth Tower in London, home to Big Ben. Credit: Pol Fernandez
In animals, some of the largest genomes include the marbled lungfish (Protopterus aethiopicus) at 129.90 Gbp and the Neuse River waterdog (Necturus lewisi) at 117.47 Gbp. In stark contrast, six of the largest-known eukaryotic genomes are held by plants, including the European mistletoe (Viscum album) at 100.84 Gbp.
Surprisingly, having a larger genome is usually not an advantage. In the case of plants, species possessing large amounts of DNA are restricted to being slow growing perennials, are less efficient at photosynthesis (the process by which plants convert the sun's energy into sugars) and require more nutrients (especially nitrogen and phosphates) to grow and compete successfully with their smaller-genomed neighbors. In turn, such effects may influence the ability of a plant to adapt to climate change and their risk of extinction.
Dr. Ilia Leitch, Senior Research Leader—Character Evolution, at RBG Kew, says, "Who would have thought this tiny, unassuming plant that most people would likely walk past without notice, could bear a world-beating record in genome size.
"Compared to other organisms, plants are incredibly diverse when viewed at the DNA level, and that should make us pause to think about their intrinsic value in the wider picture of global biodiversity. This discovery also raises many new and exciting questions about the upper limits of what is biologically possible, and we hope to solve these mysteries one day."
Adam Millward, Managing Editor of Guinness World Records, says, "To think this innocuous-looking fern boasts 50 times more DNA than humans is a humbling reminder that there's still so much about the plant kingdom we don't know, and that record holders aren't always the showiest on the outside."
#Youtube#Biology#Plants🪴 🌱#Animals 🐫🐪 🦔 🦓 🦒🐘 🐎 🦁 🐆 🐅 🦌 🐁 🐓#Molecular & Computational Biology#Fern#Genome 🧬#Organism#Phys.Org
0 notes
Text
TECH TALK : Future Technologies and Applications Driving Advanced Computing Landscapes
16 November 2023: Thank you W.Media – Global for invitation Delivered Principal #Keynote #TechTalk at “Thailand Cloud and Data Centre Convention 2023” on Research Advancements with Emerging #Deeptech Industry Applications, Information Intelligence, Interdisciplinary Practices and #AdvanceComputing Architectures will drive future landscape of #Cloud, #Datacenter and ICT industry . Dr. Saurabh…

View On WordPress
#Advanced Computing#Astronomy#Brain Research#ChatGPT#Climate Modelling#COP28#Deeptech#Design Simulations#Financial Modelling#Genetic engineering#Genome Sequencing#GPU#Human genome#Industrial Automation#Nano Material#Quantum Computing#space research#Supply chain Management
0 notes
Text

Horses are among the world’s most elite athletes: When galloping, they can consume twice as much oxygen per kilogram as the fittest humans. All that oxygen supercharges horses’ cells’ energy-producing compartments as they crank out ATP, the chemical needed to power their impressive muscles. But making so much cellular fuel so quickly comes with a catch: the manufacture of pernicious byproduct molecules called reactive oxygen species (ROS), which can wreak havoc in cells.
How horses dealt with this biological trade-off and evolved into premier endurance athletes has long intrigued biologists. Researchers report today in Science that they have uncovered a big part of it, identifying a key mutation that lets horses safely produce so much ATP. The trait helped pave the way for horses to go from dog-size critters millions of years ago to the high-endurance athletes we know today.
The study’s detailed molecular work makes it “exceptional,” says José Calbet, an expert on the cellular responses to exercise at the University of Las Palmas de Gran Canaria who wasn’t involved with the study.
The mutation in question occurs in the gene that encodes a protein called KEAP1, which acts as a biochemical bouncer, binding to a different protein called NRF2 to prevent it from entering the cell’s nucleus, where it would otherwise activate stress-response genes that help blunt cell damage.
But ROS can help NRF2 sneak in by causing KEAP1 to release its bind on the protein, allowing it to enter the nucleus and trigger the cell’s stress-response genes.
Johns Hopkins University ophthalmologist and clinician scientist Elia Duh, a senior author of the new study, didn’t set out to study horses. Initially, Duh was interested in the KEAP1-NRF2 system because its role in activating stress-response genes makes it a tempting target for treating inflammation—and aging-related conditions, such as blinding retinal diseases, irritable bowel syndrome, and neurodegeneration.
Duh wondered whether any insights could be gleaned from studying the evolution of these proteins in different animals. So, he teamed up with Gianni Castiglione, an evolutionary biologist and biochemist at Vanderbilt University. Together, they scanned hundreds of vertebrate genomes looking for notable mutations to the gene for KEAP1.
The team’s genomic work revealed birds had almost completely lost the gene, presumably an adaptation to the extreme demands of flight. When they looked in horses, researchers noticed what initially appeared to be a DNA sequence that encoded an unusually short—and therefore presumably nonfunctional—version of the KEAP1 protein. But when Duh’s and Castiglione’s team grew horse cells in culture, it discovered the protein was very much there and working. “Naturally, I was worried I was doing something wrong,” Castiglione says. “Then one day, a light bulb went off.”
As it turns out, the computer algorithm scientists had used to scan the horse genome had made a mistake. The algorithm had spotted a specific kind of mutation in the part of the KEAP1 gene that changed the messenger RNA from CGA—which codes for the amino acid arginine—to UGA, which is what’s known as a “stop codon.”
Normally, the cellular machinery interprets UGA as a sign to stop translating the RNA into a protein. But instead, the horses’ genetic machinery recodes the stop codon into a different amino acid, cysteine, causing it to ignore that order. This phenomenon, known as a stop codon read-through, is common among viruses but rare in multicellular organisms.
“The identification of this evolutionarily significant UGA recoding event represents a potentially seminal finding, offering a model for uncovering other yet-unidentified cases of stop codon read-through,” says Hozumi Motohashi, a biologist at Tohoku University who has studied KEAP1 and NRF2.
That the replacement is a cysteine is particularly notable, Castiglione says. KEAP1 senses cellular stress through its cysteines, which contain sulfur atoms whose reactions with ROS, induce the chemical changes that cause KEAP1 to let go of NRF2. The mutation the researchers had identified adds another place on KEAP1 for ROS to interact, which makes the protein more sensitive to stress—and lets horse cells respond much faster to the cellular stress of intense exercise. “It does make complete sense [that] by introducing another cysteine, another sulfur, you would have heightened sensitivity,” Castiglione says.
What’s more, this tweaking of KEAP1 is a “[key] genetic component to the puzzle of the evolution of horses,” Duh says. “Once they figured out how to run, they could occupy all kinds of ecological niches,” Castiglione adds.
The finding could also point the way toward new kinds of drugs to treat diseases by targeting the specific parts of the KEAP1 protein that help horses hoof it. “By looking at what evolution has figured out, we know this is a viable strategy,” Castiglione says.
Source
834 notes
·
View notes
Note
Following you is so amazing because I could just be reading an already random or normal post and directly below it is a computer voice saying letters and then a picture of a snail
String identified: gaagcactagaaaaatacttactcagttatactaa
Closest match: Cabera pusaria genome assembly, chromosome: 9 Common name: Common White Wave

(image source)
#tumblr genetics#genetics#biology#science#asks#sent to me#yourcrazyboyokris#bugs#insects#moths#common white wave
790 notes
·
View notes
Text
Researchers develop innovative method to compress genomic datasets for efficient local storage

- By InnoNurse Staff -
Cornell researchers have developed a new method to compress massive genomic datasets, enabling them to be stored on local computers rather than in costly and cumbersome cloud storage.
The Genotype Representation Graph (GRG) method reduces data sizes from hundreds of terabytes to gigabytes by using graph-based representations of genotypes, capturing relationships through shared genetic mutations.
This innovative approach not only compresses data compactly but also allows direct computations on the compressed data, eliminating the need for decompression.
The GRG method could enhance scalability, lower computational costs, and enable new types of analyses previously unfeasible with traditional data formats.
Published in Nature Computational Science, the method addresses the challenges of analyzing biobank-scale whole-genome data.

Image: The GRG data structure's biological significance and interpretation. Credit: Nature Computational Science (2024). DOI: 10.1038/s43588-024-00739-9.
Read more at Cornell University
0 notes
Text
Alex Shalek named director of the Institute for Medical Engineering and Science
New Post has been published on https://thedigitalinsider.com/alex-shalek-named-director-of-the-institute-for-medical-engineering-and-science/
Alex Shalek named director of the Institute for Medical Engineering and Science


Alex K. Shalek, the J. W. Kieckhefer Professor in the MIT Institute for Medical Engineering and Sciences (IMES) and Department of Chemistry, has been named the new director of IMES, effective Aug. 1.
“Professor Shalek’s substantial contributions to the scientific community as a researcher and educator have been exemplary. His extensive network across MIT, Harvard, and Mass General Brigham will be a tremendous asset as director of IMES,” says Anantha Chandrakasan, chief innovation and strategy officer, dean of the School of Engineering, and the Vannevar Bush Professor of Electrical Engineering and Computer Science. “He will undoubtedly be an excellent leader, bringing his innovative approach and collaborative spirit to this new role.”
Shalek is a core member of IMES, a professor of chemistry, and holds several leadership positions, including director of the Health Innovation Hub. He is also an extramural member of MIT’s Koch Institute for Integrative Cancer Research; a member of the Ragon Institute of Mass General, MIT, and Harvard; an institute member of the Broad Institute of MIT and Harvard; an assistant in immunology at Mass General Brigham; and an instructor in health sciences and technology at Harvard Medical School.
The Shalek Lab’s research seeks to uncover how communities of cells work together within human tissues to support health, and how they become dysregulated in disease. By developing and applying innovative experimental and computational technologies, they are shedding light on a wide range of human health conditions.
Shalek and his team use a cross-disciplinary approach that combines genomics, chemical biology, and nanotechnology to develop platforms to profile and control cells and their interactions. Collaborating with researchers across the globe, they apply these tools to study human diseases in great detail. Their goal is to connect what occurs at a cellular level with what medical professionals observe in patients, paving the way for more precise ways to prevent and treat diseases.
Over the course of his career, Shalek’s groundbreaking research has earned him widespread recognition and numerous awards and honors. These include an NIH New Innovator Award, a Beckman Young Investigator Award, a Searle Scholar Award, a Pew-Stewart Scholar Award, an Alfred P. Sloan Research Fellowship in Chemistry, and an Avant-Garde (DP1 Pioneer) Award. Shalek has also been celebrated for his dedication as a faculty member, educator, and mentor. He was awarded the 2019-20 Harold E. Edgerton Faculty Achievement Award at MIT and the 2020 HMS Young Mentor Award.
Shalek received his bachelor’s degree in chemical physics from Columbia University and his master’s and PhD in chemical physics from Harvard University. Prior to joining MIT’s faculty in 2014, he was a postdoc at the Broad Institute.
Shalek succeeds Elazer Edelman, the Edward J. Poitras Professor in Medical Engineering and Science, who has led IMES since April 2018.
“I am grateful to Professor Edelman for his incredible leadership and service to IMES over the past six years,” says Chandrakasan. “His contributions to IMES have been invaluable, and we are thankful for his dedication and vision during his tenure as director.”
#approach#Biology#Broad Institute#Cancer#career#Cells#chemical#chemistry#collaborative#Community#computer#Computer Science#course#Disease#Diseases#Edgerton#engineering#experimental#Faculty#genomics#harvard#Harvard-MIT Health Sciences and Technology#Health#Health sciences and technology#how#human#Human health#immunology#Innovation#Institute for Medical Engineering and Science (IMES)
0 notes
Text
The Weird Human Sences
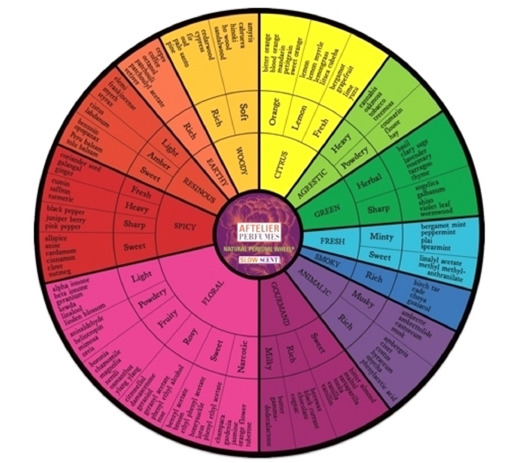
Well Known Facts
It is rather strange that the nature gave only 5 senses to the human beings. As everybody knows these senses are: Dactyl -to feel the touch and vibrations, Smell-to feel odors, aromas, Taste-to feel the tastes, Vision-to view the environment and feel the light and colors, Hearingto hear the sounds and noises.
From physical point of view
The dactyl feeling is a transmission from sensitive nerves of our hands (predominantly, but also by skin, body and legs) to the brain, where these feelings are transformed into brain impulses. The range of this sense is between 10-15Hz to more than 20 000Hz depending on the amplitudes of the vibrations. By this sense is also possible to feel temperature variations is a very narrow band (-50 0-60 0C to +70 0-80 0C). The taste feeling covers: bitter, sweet, hot, sour and salty and a small amount of combinations - at all 5 tastes.
The hearing presents the ability to hear sounds in the sound band between the infrasound (16Hz) to the ultra sounds (20 000Hz). This is relatively narrow band of the sounds hearable in the nature. The lack of detection abilities for the infrasound can be dangerous, because the infrasound has negative consequences to the human body and physics. The same is valid for the ultrasounds. The visible abilities are also in an extremely narrow band of electromagnetic spectrum for detection by the human eye-from about 380 nm (for the infrared light) to 750nm (for the ultraviolet light).
Read More About This Article: https://crimsonpublishers.com/oabb/fulltext/OABB.000565.php
Read More About Crimson Publishers: https://crimsonpublishers.com/oabb/index.php
#biostatistics journals#statistical theory#clinical trail#crimsonpublishers#Genetic Linkage#Computational Biophysics#Systems Biology#Structural genomics
0 notes